Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Moriyama, Hirotake*
JNC TJ8400 2000-050, 47 Pages, 2000/03
In support of the safety assessment of geologic disposal of high levcl radioactive wastes, the solubility of transuranium elements was studied. The solubility of PuO
 xH
xH O was measured undcr a reducing condition, and the solubility product K
O was measured undcr a reducing condition, and the solubility product K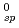 and the stability constant
and the stability constant 
 of Pu(OH)
of Pu(OH) were obtained. The obtained K
were obtained. The obtained K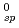 value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO
value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO 3
3  xH
xH O was measured under an oxidizing condition and the solubility product K
O was measured under an oxidizing condition and the solubility product K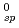 was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.
was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.